Skin Cancer Surgical Audit
2023-2025 Triennium
The 4Cyte Pathology Skin Cancer Surgical Audit has been designed by doctors for doctors with a specialist interest in skin cancers. Our Skin Cancer Audit will help doctors analyse their skin cancer diagnostic and surgical management skills to improve the health outcomes. At 4Cyte Pathology, we offer a comparative review of your personal findings against your peers and the wider GP cohort.
Why 4Cyte Skin Cancer Surgical Audit?
- Our Skin Cancer Surgical Audit was developed after conducting needs assessment with GPs, learning objectives are measurable and relevant to general practice.
- You can compare your preliminary diagnosis with confirmed histological diagnosis and identify areas where improvements can be made in diagnostic process.
- Your Skin Cancer Surgical Audit reports will be delivered online and will be accessible at any time.
- All your patients' details will be deidentified for audit purposes to protect privacy of patients.
How to Participate
Registration
Complete our registration form and return your completed form to education@4cyte.com.au or fax to +61 2 8069 0601. Upon acceptance of the application, a username and password will be issued to access the 4Cyte online CPD portal.
Data collection
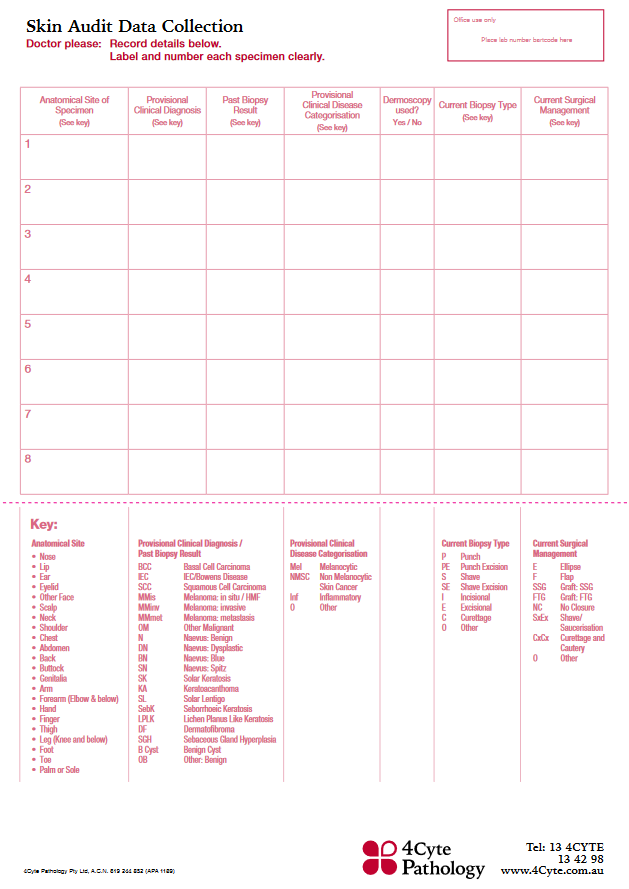
Minimum 30 samples are required to be part of this audit. Send your skin specimens using our dedicated Skin Audit Request Forms. Or you can order printed forms from your practice's 4Cyte representatives.
It is imperative that clinicians complete both sides of the Skin Audit Request Form for specimens to be included in the Audit. Please use the KEY found on the back of the Skin Audit Request Form as a guide to ensure the necessary data is collected for accurate audit reporting.

Receive your Audit Reports
Every year, two Statistical Interval Reports will be uploaded to your 4Cyte CPD Account. In this report, all your data will be presented in graphs and tables for easy interpretation. These reports will contain peer group comparisons, and will assist you in analysing your findings against your peer groups and the wider GP cohort.
Twice a year, you will be provided with Episode Summary Reports. These will include all your patient data recorded in the audit database for that audit period. This will assist to review and compare your findings and review patient managment.
Submit your Evaluation Questionnaire
At the end of the year, review the reports and complete all evaluation questionnaires. This will mark the Completion of an Audit.
Your CPD Points
Your CPD hours will be uploaded by 4Cyte within 4 weeks upon completion of the audit.
Audit Outcomes & Reporting
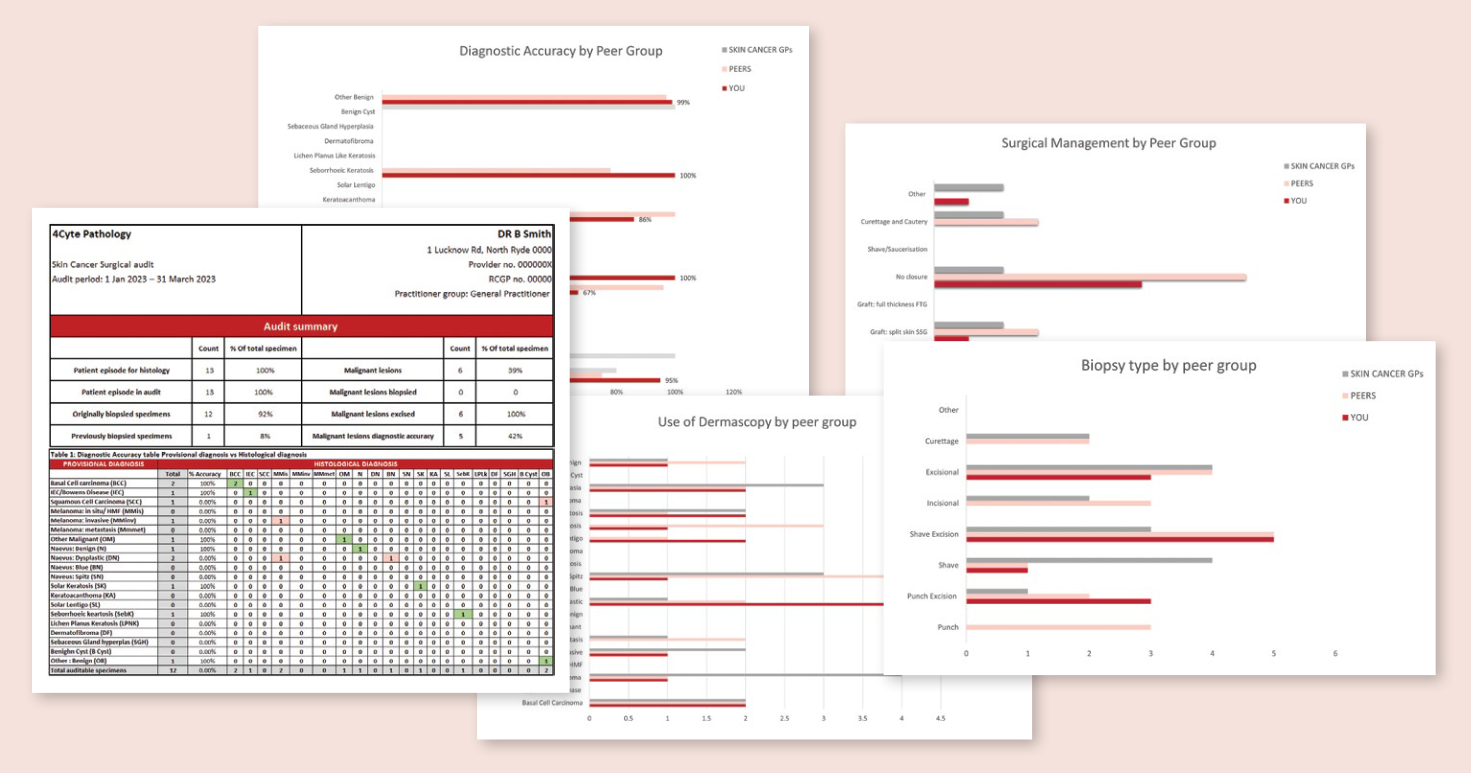
Statistical Interval Report includes:
- Total number of patient episodes referred for skin histology for the audit interval.
- Number of patient/specimen episodes in the audit.
- Originally biopsied specimens. We will exclude any previously biopsied specimen from diagnostic accuracy comparison. Information for this will be derived from past biopsy field.
- Percentage of all new lesions either biopsied or excised which have histological confirmed diagnosis of malignancy.
- Diagnostic accuracy table: your provisional diagnosis matched to the histological diagnosis; correct diagnosis will be highlighted for easy reference.
- Diagnostic accuracy graphs: your findings in comparison to peer groups and wider GP cohort.
- Diagnostic accuracy with the use of dermoscopy.
- Percentage of each biopsy type used for each skin lesions.
- Percentage of each Surgical management technique used for the biopsied or excised lesions.
Learning Objectives
By the end of this activity, you will be able to:
- Evaluate the clinical provisional diagnosis of skin lesions and their correlation with the histologically confirmed diagnosis of those skin lesions.
- Evaluate personal proficiency in skin cancer diagnosis in comparison with peers.
- Identify potential lesion types that present the participant with the opportunity for improved clinical recognition diagnosis.
- Evaluate the impact of the use of dermoscopy (or otherwise) in improving correlation of the participant's clinical provisional diagnosis with the histologically confirmed diagnosis.
- Evaluate the circumstances where dermoscopy improves clinical diagnosis.
- Compare the rate of histologically confirmed malignancy detection (i.e., BCC, SCC, IEC, Melanoma, OM) as a percentage of all new lesions.
- Evaluate the surgical management technique by understanding of the percentage of management type for biopsied and excised lesions.
- Evaluate the biopsy technique in comparison with their peers.